Reprocell and JTB commence offering
Regenerative Medicine Grade iPS Exosomes
Reprocell Inc.
JTB Corp.

Reprocell Inc. (Headquarters: Kanagawa, hereinafter "Reprocell") and JTB Corp. (Headquarters: Tokyo, hereinafter "JTB") announce that JTB becomes the general agent for handling regenerative medicine grade*1 iPS exosomes (autologous*2, allogeneic*3) (hereinafter "iPS exosomes") manufactured by Reprocell, and commences selling iPS exosomes to medical institutions and clinics through the Japan Health & Research Institute (Headquarters: Tokyo, hereinafter "Japan Health & Research Institute").
*Reprocell and JTB have been in a business partnership since October 2022.
https://www.jtbcorp.jp/jp/newsroom/2022/10/jtb-personal-ips.html
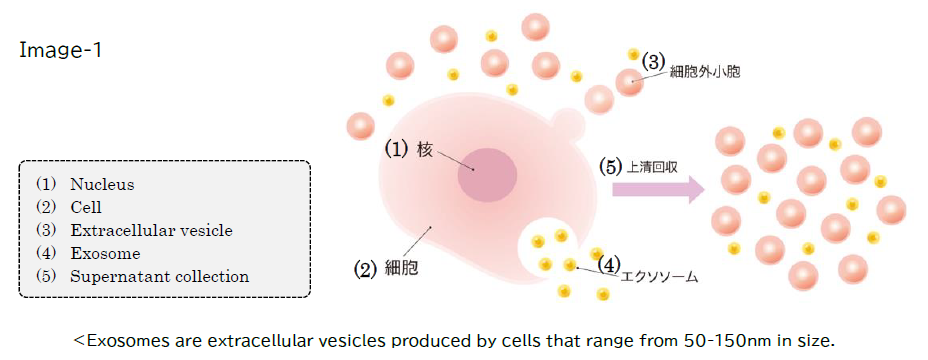
■Background and History
Exosomes are granular substances with a diameter of 50-150 nm (nanometers: one billionth of a meter) secreted by cells, playing a role in intercellular communication within the body. Currently, exosomes secreted from stem cells derived from fat, umbilical cord, and dental pulp (hereinafter "stem cell exosomes") are widely distributed for cosmetic purposes.
Reprocell and JTB have agreed to expand Japan-domestic and global sales of iPS exosomes. The global market size of exosomes is reaching 213 million USD in 2022 and it is estimated to 1,807 million USD in 2033, with the compound annual growth rate of 30%.
Citation:SDKI Analytics(https://www.sdki.jp/reports/exosome-research-products-market/106511)
●Regarding iPS exosomes(autologous & allogeneic)
JTB will provide iPS exosomes that produced by Reprocell to medical institutions and clinics. Fundamental research and animal experiment results on iPS exosomes have been reported overseas.
●Manufacturing Method and Safety
Reprocell produces a next-generation reprogramming method (mRNA method*4) without viruses and genetic introduction to create iPS cells, thus eliminating the risk of foreign factors. The cells utilized in the production of iPS exosomes are tested for viruses and bacteria to meet the regulations of regenerative medicine in Japan, the US, and Europe. The production process takes place in facilities licensed to manufacture specific cell-processed products. Furthermore, repeated purification and concentration processes thoroughly eliminate impurities, achieving high-purity regenerative medicine grade iPS exosomes.
■Overview
To promote iPS exosomes as a Japanese technology domestically and globally, and to establish a distribution system to Japanese medical institutions and clinics, the handling of 'Personal iPS*5' has been supervised by Dr. Toshio Miyata (Chairman of the Medical Corporation DEN). Test operations will commence at Mii Clinic Yoyogi (Tokyo), Shiba International Clinic (Tokyo), HAAB BEAUTY CLINIC Minami Aoyama Main Branch (Tokyo), my place beauty clinic (Osaka), and Mii Clinic Minoo (Osaka). At the same time, through JTB's patient collection and referral scheme, we aim to further expand medical inbound by selling the iPS exosome plans provided by medical institutions and clinics to foreign visitors to Japan.
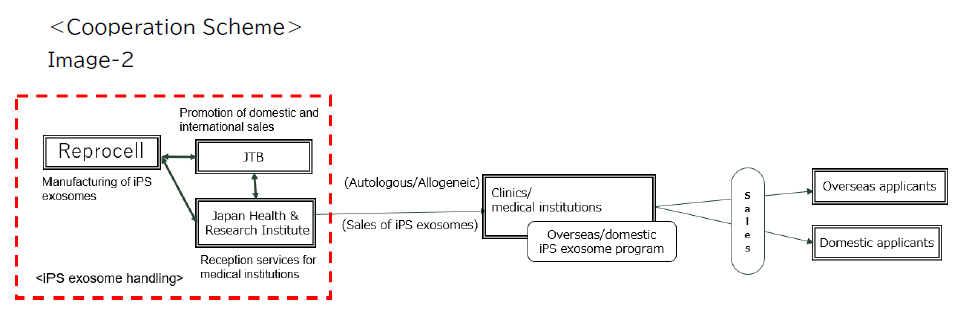
<Main Roles of Cooperation>
・Reprocell: Manufacturing iPS exosomes
・JTB: Promotion of domestic and overseas sales of iPS exosomes
・Japan Health & Research Institute: Reception and counter services for handling stores (medical institutions and clinics)
<Company Information>
Reprocell (TSE Growth: 4978) was established in 2003 as a biotech venture originating from Kyoto University and the University of Tokyo. With the world's most advanced iPS cell technology, the company's products and services are widely used in universities and pharmaceutical companies worldwide. Reprocell is also actively engaged in the development of regenerative medical products and is a leading company in the iPS cell field.
JTB aims to connect people, places, and things to create new value and foster innovation in local communities through its "Bringing People, Places and Possibilities Together"*6 Since 2008, JTB has been engaged in medical coordination business in medical inbound tourism to Japan.
■Japan Health & Research Institute
The Japan Health & Research Institute is dedicated to promoting health and recovery for the public through natural environment-based health promotion programs such as spa therapy and climate therapy, operating comprehensive health check-up centers, and advancing preventive medicine.
*1 Regenerative medicine grade: Grade based on standards for cell processing for clinical use (infection testing of cells and manufacturing facility).
*2 Regenerative medicine-grade iPS exosomes (autologous): iPS exosomes manufactured from the patient's own cells (iPS cells)
*Autologous: Manufactured from the patient's own iPS cells, requiring an application for Reprocell's Personal iPS service, which provides manufacturing and storage of individual iPS cells.
*3 Regenerative medicine-grade iPS exosomes (allogeneic): iPS exosomes manufactured from another person's cells (iPS cells)
*4 mRNA method: A method for producing iPS cells using messenger RNA (ribonucleic acid).
*5 Personal iPS: A completely new personal cell storage service utilizing technology to create iPS cells from urine or teeth. By storing one's own iPS cells, it prepares for future regenerative medicine applications, such as cell transplants without rejection reactions.
* "Personal iPS" is a registered trademark of Reprocell Co., Ltd.
*6"Bringing People, Places and Possibilities Together"is a registered trademark of JTB Corporation.
■For inquiries regarding iPS exosomes:Reprocell Inc.
info_jp@reprocell.com
■For inquiries regarding the handling of iPS exosomes for medical institutions and clinics: Japan Health & Research Institute
ips-exo@jph-ri.or.jp
Website for prospective iPS exosome dealers:
https://www.jph-ri.or.jp/medical/ips.html
■Contact for press inquiries:JTB Corp. Public Relations team
Phone: +81 3 5796 5833
